
Like other steels, they are iron and carbon alloys to which chromium and other elements are added to improve strength. For the uninitiated, stainless steel is a simple term for a material that is indestructible, does not rust and withstands all forms of corrosion.
In reality, its strength comes from a thin layer of oxide formed on its surface. This protective layer significantly slows down the oxidation process (formation of rust*) which is not the case with a non-alloyed material which can rust or corrode because its oxide layer is porous.
To be classified as stainless, a steel must contain at least 10.5% chromium and less than 1.2% carbon. Most stainless steels in use comply with standards: European (EN 10088 standard in particular) and American (AISI standard)
Stainless steels play a role in countless fields:
Everyday applications, Mechanical industry, Construction, Food industry, Chemicals, Transport, Nautical industry, Medicine, ....
* see definitions - last paragraph
Stainless steels come in two families:
(Martensitic and Ferritic) contain 12 to 30% chromium and a small percentage of nickel.
Their structure is:
- Martensitic They combine good corrosion resistance and high mechanical properties. (For example, surgical instruments and cutlery)
- Ferritic: Do not harden, are heat resistant and cheaper than chrome nickel steels (For example, architecture, kitchen utensils)
(Austenitic and Austenitic-ferritic). Their base grade contains 18% Chrome and 10% Nickel.
They contain little carbon: from 0.02% to 0.15%, their structure is:
- Austenitic*: They do not harden, are non-magnetic, are very ductile and react well to extreme temperatures.
- Austenitic-ferritic: their structure is biphasic*. Their specifications are similar to those previously encountered and they are mainly used to produce cast steels, particularly suitable for welding.
It is for their improved corrosion resistance and their high mechanical and chemical specifications that INOX SYSTEM only markets and works with austenitic grade stainless steels.
* see definitions - last paragraph
A stainless steel grade corresponds to the metal's chemical composition and not its surface appearance. Grades are selected depending on the environment and atmospheric conditions.
Austenitic steels have high hot or cold corrosion resistance in aggressive environments. This resistance is due to the presence of chromium (over 15%) which protects the surface of the alloy by passivation.
Resistance to atmospheric corrosion is then multiplied a hundredfold compared to standard steels.
Standards
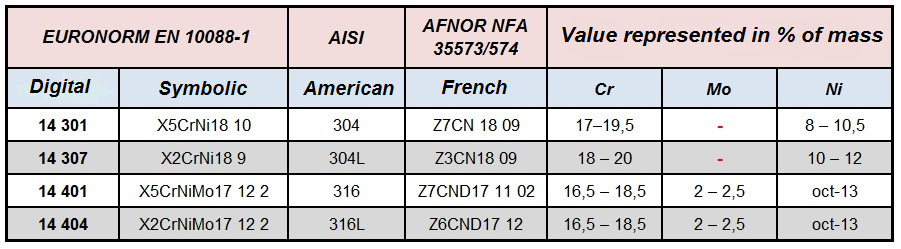
Chemical composition
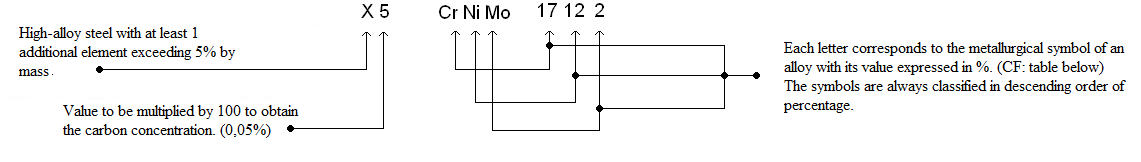
Value represented in % by weight.
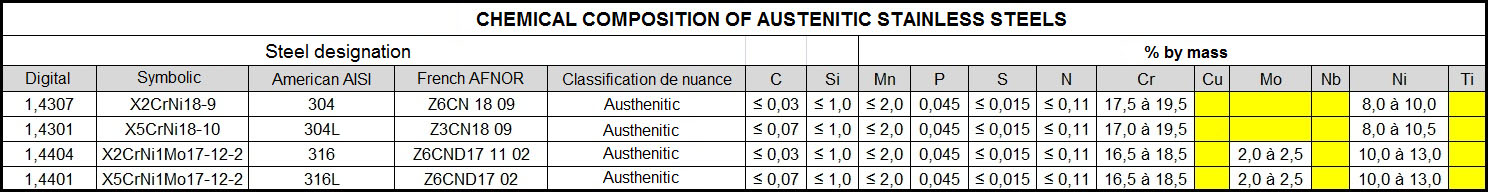
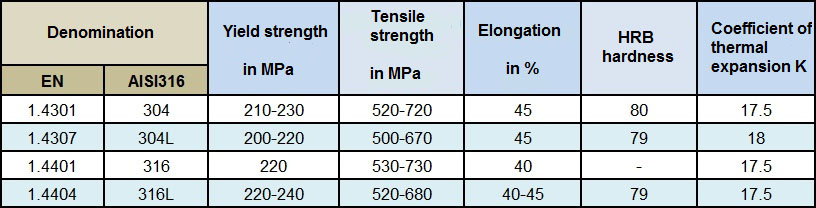
Taking type 1.4301 (304) as a basis, one can see that the mechanical properties and oxidation resistance increase when Chromium and Nickel are added:
- Chromium (Cr, C): An addition of more than 13% makes the steel stainless, but too hard.
- Nickel (Ni; N): Adding Nickel increases stability, improves corrosion resistance, ductility and facilitates use.
An addition of more than 20% makes the steel stainless but too soft.
The addition of chromium and nickel in suitable proportions guarantees good technical and mechanical specifications for possible machining.
The addition of Tungsten and Molybdenum (Mo, D) improves wear and heat resistance and increases pitting corrosion resistance in aggressive environments as well as passivation film stability.
* see definitions - last paragraph
304 and 304L grade stainless steel
Alloys without added molybdenum, they have good ambient and inter-granular corrosion resistance. They have good weldability, and are suitable for use in the food industry (kitchen equipment, restaurants, cutlery) and in less aggressive environments such as boiler making and piping.
316 and 316L grade stainless steel
These alloys have low carbon content and a reliable addition of molybdenum, which gives them very good pitting corrosion resistance and makes them suitable for hot working. They are suitable for very aggressive environments, in particular chlorinated and marine environments.
We now have a material analyser (X-ray fluorescence principle),
and we regularly check arrivals to guarantee faultless quality.

Austenitic stainless steels are vulnerable to surface corrosion. The most frequent is the appearance of rust, either resulting from the presence of external metallic dust, or from simple scratches or rubbing against parts of a different type.
In addition to rust, there are three other types of electrochemical corrosion: intra-granular corrosion*, pitting corrosion* and stress corrosion*.
Such marks can easily be removed using a stainless steel cleaner, such as WICHINOX ®
Stainless steel: high-alloy corrosion agent resistant steel
Austenitic steel: steel that does not harden and is non-magnetic. It combines high corrosion resistance with good deformability for easy shaping. They are the most commonly used stainless steels.
Alloy: a product resulting from the mixture of a metal with other metallic or non-metallic elements.
Austenite: micrographic constituent of steels. Existing from 900°C to 1300°C. Mixture of iron and dissolved carbon
Intra-granular corrosion: 3 conditions: at least 0.035% carbon, acidic and oxidizing external environment, kept at a temperature between 400 and 800°C.
Pitting corrosion: accidental presence of metallic dust, which, in a humid environment, creates a battery system that corrodes the steel.
Stress corrosion: a rare result, seen when the subject is exposed to a corrosive environment such as: impure water, chloride solutions or soda. The element then very quickly becomes unusable.
Breaking load: is the static load that the material provides until total breakage of a sample in Newton/mm² when new.
Work load: indicates the static load value for which the product will continue to operate without wear.
Ductility: property of being able to be stretched without breaking
Elastic limit: is the maximum load limit to be applied to a specimen before it is deformed.
Passivated: steel made less vulnerable to corrosion by treatment
Quenching: rapid cooling of a metallurgical material or glass to obtain a stable structure at room temperature
Rust: alteration of iron and steel in the presence of oxygen and humidity.